|
|
Cathode and Anode are commonly used terms in the context of electrochemistry, specifically in electrochemical cells like batteries and electrolytic cells. An anode is a negative or reducing electrode that releases electrons and oxidizes during an electrochemical reaction whereas a Cathode is a positive or oxidizing electrode. In this article, we will learn about, Cathode, Anode, their differences, and others in detail. What are Cathode and Anode?Cathode and Anode are the basic terms of electrochemistry that are used to denote the two positive and negative parts of any electrochemical cell or battery. Now let’s learn about Cathode and Anode in detail. Cathode Definition
Cathode is a crucial component in various electrochemical processes, playing a key role in facilitating reduction reactions. In the context of batteries, the cathode is the electrode where electrons are accepted from the external circuit during a spontaneous electrochemical reaction. The significance of the cathode extends beyond its role in electrochemistry. It serves as a critical element in technologies such as cathode-ray tubes (CRTs) and certain types of diodes. Anode Definition
Anode is another important of an electrochemical systems, representing the electrode where oxidation reactions occur. In the context of batteries, the anode is the site where electrons are released to the external circuit during a spontaneous electrochemical reaction. This release of electrons is typically accompanied by the oxidation of ions or molecules, resulting in the development of a negative charge. In electronic devices like diodes, the anode is the electrode where current enters, and it plays a crucial role in regulating the direction of electric flow. Charges on Cathode and AnodeIn electrochemical cells,
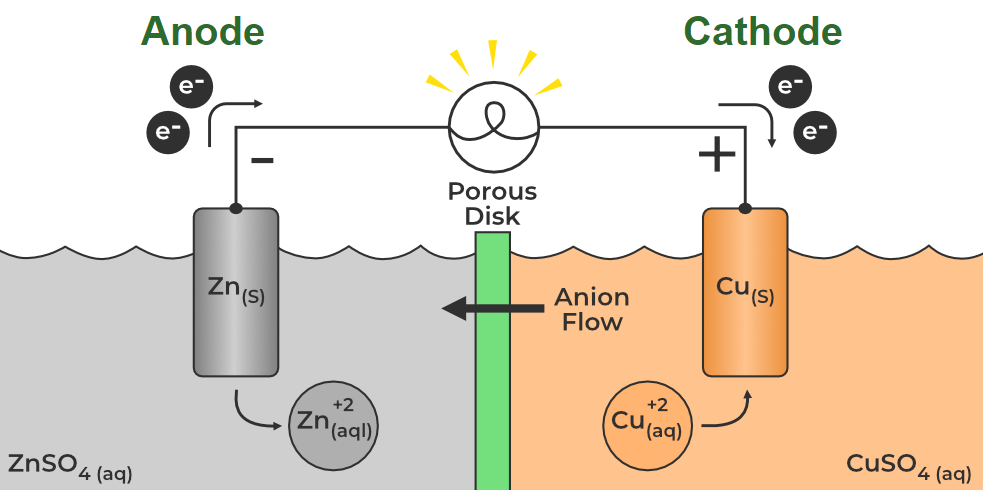
Cathode and Anode MeaningCathode and Anode are terms used to identify the two electrodes in an electrical device, such as a battery or an electrolytic cell. Cathode and Anode in an Electrochemical Cell is shown in the image added below, Anode and Cathode in Electrolytic CellIn electrolysis, the terms “anode” and “cathode” are used to describe the two electrodes immersed in an electrolyte, which is a solution that conducts electricity. Cathode in Electrolysis
Anode in Electrolysis
Cathode and Anode of a DiodeIn the context of a semiconductor diode, such as a typical silicon or germanium diode used in electronic circuits, the terms “cathode” and “anode” refer to the two terminals of the diode. Here’s how these terms are applied to a diode: Cathode of a Diode
Anode of a Diode
Anode Vs CathodeThe differences between cathode and Anode are added in the table below,
Conclusion on Cathode and AnodeCathode and anode are essential in understanding the behavior of diodes in electronic circuits.
This fundamental understanding of the cathode and anode plays a pivotal role in the proper integration of diodes within circuits, ensuring their intended functionality during both forward and reverse biasing. Whether rectifying current or serving in signal processing, diodes’ distinct terminal characteristics make them indispensable components in the realm of electronics. Read More, Cathode and Anode QuestionsQ1. Identify the cathode and anode in a galvanic cell with the reaction Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
Q2. In a battery, if electrons flow from the copper electrode to the zinc electrode, which electrode is the cathode?
Q3. Describe the electron flow and conventional current direction in a simple circuit with a battery and a light bulb.
Q4. In a vacuum tube, if electrons are emitted when the cathode is heated, what type of electrode is the cathode?
Cathode and Anode-FAQsWhat is Charge of Anode and Cathode?
How to Determine which Electrode is Cathode and Anode?
Is A Cathode Positive or Negative?
What are Materials Used for Anode and Cathode?
What is Function of a Salt Bridge?
|
||||||||||||||||||||||||
Reffered: https://www.geeksforgeeks.org
| Class 12 |
Type: | Geek |
Category: | Coding |
Sub Category: | Tutorial |
Uploaded by: | Admin |
Views: | 16 |