|
|
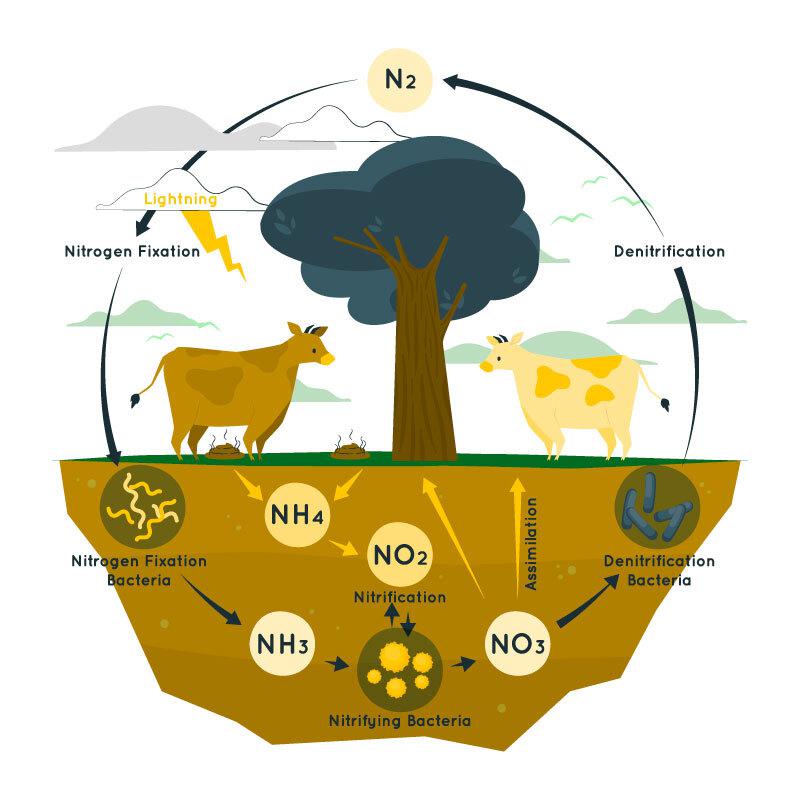
Nitrogen fixation is the process of converting atmospheric nitrogen (N2) into ammonia (NH3) or related compounds. This process is primarily carried out by nitrogen-fixing bacteria, either in symbiotic relationships with plants or free-living in the soil. Abiotic processes, like lightning and industrial methods, can also fix nitrogen. All biological reactions involving the process of nitrogen fixation are catalyzed by enzymes called nitrogenases. Nitrogen fixation is important for enriching soil fertility and providing the nitrogen needed for plant growth, making it a key component of the nitrogen cycle and an essential element for life on Earth. Table of Content What is Nitrogen Fixation?Earth’s atmosphere is made up of 78% nitrogen, 21% oxygen, 0.93% argon, 0.04% carbon dioxide, and trace quantities of other gasses. Nitrogen fixation is a biological process that converts atmospheric nitrogen gas (N2) into ammonia (NH3) or related compounds that can be used by plants and other organisms. This process is mainly carried out by nitrogen-fixing bacteria, such as Rhizobium in legume root nodules or free-living soil bacteria like Azotobacter. In symbiotic relationships, plants supply carbohydrates to the bacteria, which, in turn, provide ammonia to the plant. Abiotic nitrogen fixation occurs through lightning and the Haber-Bosch process in industrial settings. The ammonia produced increases soil fertility, supporting plant growth. Nitrogen fixation plays an important role in maintaining the nitrogen balance in ecosystems and is important for agriculture, providing the basis for nitrogen-containing fertilizers. Nitrogen Fixation DiagramThe diagram below shows the process of nitrogen fixation:
Different Ways of Nitrogen FixationNitrogen is a limiting nutrient in ecosystems, and its availability is important for the growth and productivity of plants. There are certain bacteria and some natural phenomenon that help in nitrogen fixation so that the nitrogen can be used by the plants. The different ways of nitrogen fixation are as follows: Biological Nitrogen FixationSome bacteria have capacity of converting atmospheric nitrogen to ammonia. This process is called biological nitrogen fixation. The enzyme nitrogenase converts dinitrogen to ammonia. Nitrogen-fixing bacteria may be symbiotic. Some of the nitrogen fixers are Azotobacter, Beijernickia, Rhodospirillum, cyanobacteria, etc. Examples of symbiotic nitrogen fixers are Rhizobium (in the root nodules of legumes). Symbiotic Nitrogen FixationA types of microorganisms called Rhizobium, help in nitrogen fixation. These microscopic organisms live in the roots of leguminous plants (e.g., pea and beans plants) and use specific kinds of enzymes, they help in fixing nitrogen in the soil and supply the plant with ammonia. In return, the plant provides carbohydrates to the bacteria. This type of nitrogen gets dissolves in the soil,and plants absorb the changed nitrogen from the soil. This is the purpose for farmers carrying out crop rotation, where leguminous plants help to refill nitrogen content in the soil without the need of fertilizer. Nitrogen fixation by microscopic organisms is an example of the symbiotic relationship between Rhizobium and leguminous plants. While microscopic organisms fix nitrogen in the soil, plants give them food. Non-symbiotic Nitrogen FixationFree-living nitrogen-fixing bacteria, such as Azotobacter and Clostridium, can be found in the soil. These bacteria fix nitrogen without a direct symbiotic relationship with plants. They take atmospheric nitrogen and convert it into ammonia or related compounds, which can then be used by nearby plants. Abiotic Nitrogen FixationNitrogen Fixation done by abiotic factors such as via lightning or Haber-Bosch Process. Nitrogen Fixation by LightningLightning is a natural phenomenon that can generate enough energy to break the strong nitrogen-nitrogen triple bond (N2) in the atmosphere. This results in the formation of nitrogen oxides (NO and NO2), which can be dissolved in rainwater and deposited into the soil, providing a source of fixed nitrogen. The contribution of lightning in the nitrogen fixation is small and they save plants from the deficiency of nitrogen. Haber-Bosch ProcessThe Haber–Bosch process also called Haber process, is the main industrial procedure for the production of ammonia. In the Haber process, the atmospheric nitrogen (N2) is converted to ammonia (NH3) by reacting it with hydrogen (H2) at high-temperature and high-pressure. This process uses natural gas as a hydrogen source and air as a nitrogen source. The Haber Process can be used to make fertiliser from ammonia used in agriculture. Nitrogen MetabolismNitrogen metabolism is mainly based on the recycling of ammonia (NH3) into the neutral or charged form ammonium ion (NH4+). It begins with the assimilation of inorganic nitrogen (usually as ammonium or nitrate) into organic molecules like amino acids, which are the building blocks of proteins. In the living beings excess nitrogen is stored as urea or uric acid and excreted. In plants, nitrogen metabolism is important for growth and development. In humans, it’s essential for protein synthesis and maintaining nitrogen balance. The main part of nitrogen metabolism is the Nitrogen Cycle.
FAQ’s – Nitrogen Fixation1. What is Nitrogen Fixation?
2. What is Nitrification Process?
3. Nitrogen-fixing Organisms Which can be Free as well as Symbiotic is?
4. What is Denitrification?
5. What is an Example of Nitrogen Fixation?
|
Reffered: https://www.geeksforgeeks.org
| Class 8 |
Type: | Geek |
Category: | Coding |
Sub Category: | Tutorial |
Uploaded by: | Admin |
Views: | 12 |