|
|
The branch of chemistry that deals with the molecules involved in living things is called biochemistry. Carbohydrates, proteins, vitamins, and nucleic acids are some of the major components of our body. These are collectively called biomolecules. A biomolecule is sometimes associated as a biological molecule, a term that refers to molecules found in living objects that are important for one or additional biological processes, analogous to cell division, morphogenesis, or development. Large macromolecules (or polyanions) similar to proteins, carbohydrates, lipids, and nucleic acids, as well as primary metabolites, secondary metabolites, and smaller moieties, similar to natural products, are all examples of biomolecules. Natural material is the more broad term for this type of material. Biomolecules are essential factors of living organisms. While endogenous biomolecules are made within the organism, organisms usually require external biomolecules, such as specific nutrients, to be present.
Table of Content What are Monosaccharides?Monosaccharides are polyhydric aldehydes and ketones that cannot be hydrolyzed into simple carbohydrates. Monosaccharides are classified as:
Predicated on the composition of carbon atoms, they’re else broke down as triose, tetraose, pentose, hexose, heptose, etc. Thus, when nominating these monosaccharides, the prefix indicates the composition of carbon atoms similar as tetra- (4), Penta- (5), Hexa- (6), hepta- (7), etc. is comprehended in the expression aldose or is done. ketos. For illustration, an aldopentose means that it’s an aldehyde carbohydrate- bearing five carbon atoms. Also, ketohexose means a ketone holding six carbon atoms. Most monosaccharides are found in nature. They are colorless, crystalline solids, soluble in water, and have a sweet taste. These are quite stable and are not hydrolyzed. They sizzle when heated and give off a distinctive odor. Optionally active.
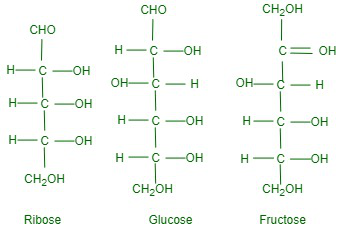
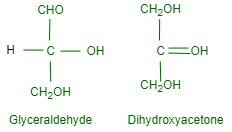
Structures of MonosaccharidesThe simplest monosaccharides are trioses such as glyceraldehyde and dihydroxyacetone, both of which have the molecular formula C3H6O3, glyceraldehyde is aldose while dihydroxyacetone is ketose as shown below:
The majority of famed monosaccharides are ribose, C5H10O5, glucose C6H12O6, and fructose C6H12O6. Ribose is aldopentose, glucose is aldohexose while fructose is ketohexose.
D- and L- DesignationSugars are divided into two families: the D-family and the L-family which have definite configurations. These configurations are indicated with respect to glyceraldehyde as the standard. Glyceraldehyde can be presented in two forms: In the D-configuration -OH is attached to the carbon adjacent to -CH2OH while in the L-configuration – OH is attached to the carbon adjacent to the -OH on the left. The sugar is referred to as D- or L- depending on whether the configuration of the molecule is related to D-glyceraldehyde or L-glyceraldehyde. It has been found that all naturally occurring sugars are related to the D-chain D-glucose, D-ribose, and D-fructose.
Where can we find Glucose?Glucose can be found naturally in many fruits, honey, and vegetables. It is also present in large amounts in sweet fruits like grapes and in honey. Presence of Asymmetric Carbon AtomsOn careful examination of monosaccharide molecules, we see that they contain one or more chiral carbon atoms. For example, glucose has four chiral carbon atoms (carbons 2, 3, 4, and 5). We know that if the molecule has n chiral carbon atoms, it will have 2n optical isomers. Therefore, glucose has 24 or sixteen optical isomers. Three of these are sixteen aldohexoses which are D-glucose, D-galactose, and D-galactose, D-mannose.
It may be noted that in all three of these molecules, the configuration of C-5 is the same (-OH on the right) and hence, they belong to the D-family. Examples of MonosaccharidesGlucoseGlucose occurs in nature in an autonomous as well as a related fashion. It is present in sweet fruits and honey. Ripe grapes contain about 20% glucose and that is why it is also known as grape sugar. Glucose in related form is substantial in polysaccharides such as cane sugar and starch and cellulose. Sample Questions on MonosaccharidesQuestion 1: Why are carbohydrates usually optically active? Answer:
Question 2: What are monosaccharides? Answer:
Question 3: Explain what is meant by the pyranose structure of glucose? Answer:
Question 4: What are the structural features of reducing sugars? Answer:
Question 5: What are the two functions of carbohydrates in plants? Answer:
Preparation of Glucose
C6H22O11(sucrose) + H2O → C6H12O6(glucose) + C6H12O6(fructose)
(C6H10O5)(starch) + nH2O → C6H12O6(glucose) In this process, an aqueous solution of starch obtained from corn is acidified with dilute H2SO4 then it is heated in an autoclave under 2-3 atm pressure steam. When hydrolysis is complete, the liquid is neutralized with sodium carbonate to a pH of 4–5. The resulting solution glucose Xe is concentrated under reduced pressure to obtain crystals of glucose. FructoseFructose is found in fruits and is called fruit sugar. It is also present in honey and sweet fruits along with glucose. In the combined state, it is also present in disaccharides (sucrose) and polysaccharides (insulin). It is obtained by hydrolysis of cane sugar with dilute H2SO4 with glucose. C12H22O11 + H2O → C6H12O6(D- glucose) + C6H12O6(D- fructose) This solution containing equal molecules of D-glucose and D-fructose is called invert sugar. Check: Monosaccharides – FAQsHow do monosaccharides function in the body?
What are common examples of monosaccharides?
How are monosaccharides structurally characterized?
What is the significance of the structural differences between glucose and fructose?
How do monosaccharides form more complex carbohydrates?
|
Reffered: https://www.geeksforgeeks.org
| Class 12 |
Type: | Geek |
Category: | Coding |
Sub Category: | Tutorial |
Uploaded by: | Admin |
Views: | 12 |