|
|
As we all have a decent understanding of baker’s ammonia in day to day life which is employed within the kitchen for creating tasty crispy baked cookies or crackers biscuit, it’s also employed in fast cooking. In modern chemistry, we call it chemically “ammonium bicarbonate”. The term Ammonium bicarbonate is really an old term that’s now commonly referred to and replaced with other substances like Baking powder (leavening) or baking soda (saleratus). It’s a very important point to know that baker’s ammonia is completely different from household ammonia which could be a toxic substance. What is Ammonium Bicarbonate?Ammonium Bicarbonate is an inorganic chemical compound that’s mildly basic. It consists of the ammonium cation and therefore the bicarbonate anion. Chemically it is the bicarbonate salt (HCO-3) of ammonia (NH+4). It’s a solid that has no colour and it degrades readily to ammonia, carbon dioxide, and water. Carbon dioxide: One in all the heavy colourless gas which is created by the burning of plant or animal matter. Its statement is CO2 and also acidic in nature with soluble in water. CO2 occurs naturally in the earth’s atmosphere as joined by the trace gas. Ammonia: Ammonia may be a colourless, highly irritating gas with a pungent smell and suffocating odour. Its formula is NH3. About 80% of the ammonia produced by industry is employed in agriculture as fertilizers. Ammonia is also used for the manufacturing of plastics, explosives, textiles, pesticides, dyes and other chemicals. It’s also used as a refrigerant gas, for the purification of water supplies. Water: Water is formed from hydrogen and oxygen, and it exists in gaseous, liquid, and solid states. Water is one of the foremost plentiful and essential compounds, occurring as a liquid on the planet’s surface under normal conditions. Its chemical formula is H2O with the molecular mass of eighteen. This compound has multiple names which reflect its long history, such as:
Chemical formula and Molecular formula
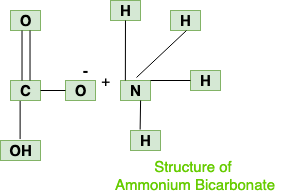
Molar Mass: It has molar mass of 79.056g/mol. Appearance and Melting Point: White crystalline solid with a melting point of 41.9°C. StructureAmmonium bicarbonate chemical structure is made up of the ammonium cation NH4+ and the bicarbonate anion HCO3–.
Occurrence Ammonium bicarbonate is present during a small quantity in nitrogenous organic matter, together with multiple other ammonium salts. It also occurs naturally as a really rare mineral referred to as “Teschemacherite”. Production The Ammonium bicarbonate is formed by combining two things that are greenhouse gas which is carbon dioxide and ammonia:
As ammonium bicarbonate is unstable thermally, the reaction solution is always kept under the cold temperature. Thus, this ends up allowing the precipitation of the merchandise as the white solid. About 100,000 tons were produced in a similar way within the year 1997. Ammonia gas sent to a solution (aqueous) of sesquicarbonate that’s a 2:1:1 mixture of, (NH4)HCO3, (NH4)2CO3 and H2O transforms it into a traditional ammonium carbonate ((NH4)2CO3), which may be obtained within the crystalline condition from a solution or preparation created at about 30 °C of temperature. On exposure to this compound to air provides off ammonia and reverts it to the ammonium bicarbonate. Salt of Hartshorn: Compositions containing carbonate of ammonium have long been known. They were once produced commercially, formerly referred to as a spirit of ammonia or salt of hartshorn. It absolutely was obtained by the dry distillation of nitrogenous organic matter like hair, horn, leather. Additionally to ammonium bicarbonate, this material contains ammonium carbamate (NH4CO2NH2), and ammonium carbonate ((NH4)2CO3). It’s sometimes called ammonium sesquicarbonate. It gives out a really strong ammoniacal smell, and on digestion with alcohol, the carbamate is completely dissolved leaving a by-product of ammonium bicarbonate. The same decomposition takes place when the sesquicarbonate is exposed to air. Physical PropertiesPhysical properties of ABC(Ammonium Bicarbonate) can be stated as following;
Chemical PropertiesChemical properties of ABC (Ammonium Bicarbonate) will be stated as follow;
NH4HCO3 = NH3+ CO2+ H2O
Uses of Ammonium BicarbonateFollowing are the key commercial and general uses of ammonium bicarbonate:
Sample QuestionsQuestion 1. What is the way to measure the concentration of the bicarbonate, after we are purging the solvent (water) with the conventional air or CO2 gas? Answer:
Question 2. Is ammonium bicarbonate toxic? Answer:
Question 3. What are the key points of ammonium bicarbonate? Answer:
Question 4. In the pH scale where does ammonium bicarbonate lie? Answer:
|
Reffered: https://www.geeksforgeeks.org
| School Chemistry |
Type: | Geek |
Category: | Coding |
Sub Category: | Tutorial |
Uploaded by: | Admin |
Views: | 11 |