|
|
Werner’s Theory of Coordination Compounds was proposed by a Swiss Chemist Alfered Werener in 1898. Werner studied the physical, chemical, and isomeric properties of several coordination compounds and postulated some theories. In this article, we will learn about, Werner’s Coordination theory, its postulates, and others in detail. Werner Coordination TheoryWerner postulated the following theories regarding Coordination Compounds after his study:
Since Werner’s Coordination Theory talks about Primary or Secondary valencies also known as Primary and Secondary Linkages, let’s learn about them in detail. Postulate of Werner’s TheoryWerner’s Theory explains that the central metal atom in the coordination compound has two types of vacancies that are,
Let’s learn about them in detail. Primary ValencyThe valencies that Metal exhibits in the production of simple salts generally Binary compounds CoCl3, NaCl, and CuSO4, are known as primary valencies. In modern terms, it refers to a Metal’s oxidation number. For example, in CoCl3, the Primary Valency of Co is 3 and the Oxidation State is +3. Features of Primary Valency
Secondary ValencySecondary Valency refers to the number of ions or groups of atoms directly to the Metal in a coordination compound. They are inside the square bracket in Coordination Compound molecular formula. For Example, in [Co(NH3)6]Cl3 the secondary valency of Co is 6 as 6 molecules of NH3 are attached to Co. They are called Metal’s Coordination Number. Features of Secondary Valency
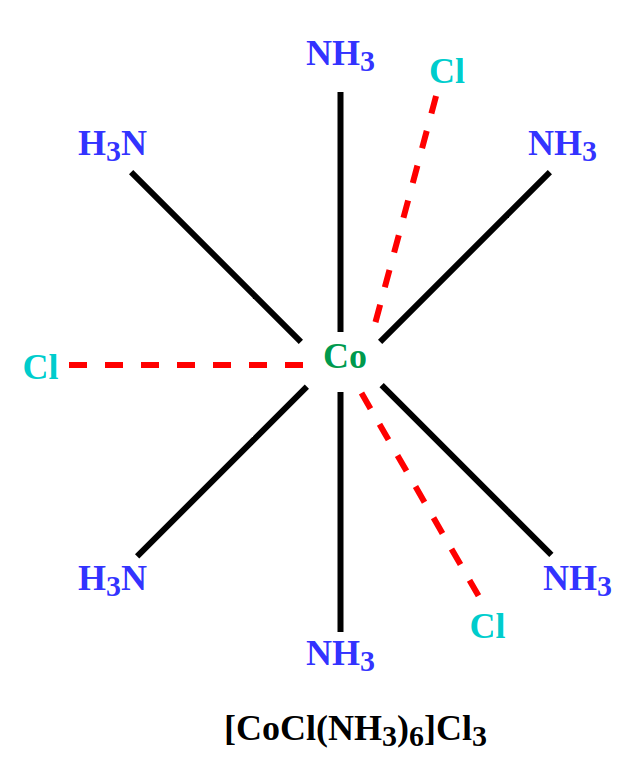
Structures of Coordination Compound based on Werner’s TheoryLet’s study the following Coordination Compounds of Cobalt on the basis of Werner’s Theory. It should be noted that as per convention, dotted lines (…..) show primary valency and thick lines ( __ ) show secondary valency [Co(NH3)6]Cl3In this, there are three primary bonds that are ionizable and are satisfied by Cl– ion also called Counter ion, which is represented outside the square bracket. The number of secondary valency is 6 which is satisfied by neutral NH3 molecules and written inside the square bracket. These six secondary valences determine the shape of the molecule. ![[Co(NH3)6]Cl3](https://media.geeksforgeeks.org/wp-content/uploads/20230614165449/Coordination-Comp-4.png)
[CoCl(NH3)5]Cl2In this, there are two primary bonds that are ionizable and satisfied by Cl– ion also called Counter ion, which is represented outside the square bracket. The number of secondary valency is 6 which is satisfied by 5 neutral NH3 molecules and 1 Cl atom and written inside the square bracket. These six secondary valences determine the shape of the molecule ![[CoCl(NH3)5]Cl2](https://media.geeksforgeeks.org/wp-content/uploads/20230614165512/Coordination-Comp-3.png)
[CoCl2(NH3)4]ClIn this, there are two primary bonds that are ionizable and satisfied by Cl– ion also called Counter ion, which is represented outside the square bracket. The number of secondary valency is 6 which is satisfied by 4 neutral NH3 molecules and 2 Cl atoms and written inside the square bracket. These six secondary valences determine the shape of the molecule. ![[CoCl2(NH3)4]Cl](https://media.geeksforgeeks.org/wp-content/uploads/20230614165545/Coordination-Comp-2.png)
[CoCl3(NH3)]In this, there are no primary bonds that are ionizable. The number of secondary valency is 6 which is satisfied by 3 neutral NH3 molecules and 3 Cl atoms and written inside a square bracket. Here all the 3 Cl atoms are non-ionizable means that they can’t be precipitated out. These six secondary valences determine the shape of the molecule. ![[CoCl3(NH3)]](https://media.geeksforgeeks.org/wp-content/uploads/20230614165418/Coordination-Comp-1.png)
Limitations of Werner’s TheoryThe limitations of Werner’s Coordination Theory are as follows:
Since all the above discussions were based on Coordination Compounds hence let’s have a glance at Coordination Compounds’ definitions and properties What are Coordination CompoundsCoordination compounds are chemical compounds composed of an array of anions or neutral molecules linked by coordinate covalent bonds to a central atom. Coordination compounds are also known as coordination complexes. The molecules or ions that are connected to the center atom are referred to as ligands (also known as complexing agents). Metal complexes are coordination compounds in which the central atom is a metallic element. In this type of coordination complex, the central tom is frequently a transition element. It should be noted that the coordination center is the central atom in these complexes. Properties of Coordination Compounds
Applications of Coordination CompoundsCoordination compounds’ unique features, make them particularly helpful in various processes and industries. Some of these coordination compound applications are listed below.
Similar to Coordination Complex, Double Salts are also formed by the combination of two or more stable compounds in a stoichiometric ratio. However, differences exist between them. Difference between Double Salt and Coordination ComplexThe basic difference between Double Salt and Coordination is that in aqueous solutions, Double Salts are totally ionizable, and each ion in the solution delivers the corresponding confirmatory test. Potash alum, for example, is a Double Sulfate. K2SO4 is the chemical formula. When Al2(SO4)3.24H2O is ionized, it produces K+, SO2-4, and Al+3 ions, which respond to the tests while In aqueous solutions, coordinate complexes are only partially ionizable. These produce a complex ion that isn’t completely ionized. Potassium Ferrocyanide is one example. [K4Fe(CN)6]. K+ and [Fe(CN)6]4- [ferro cyanide ions] are formed when it ionizes. Heteroleptic ComplexThe complex compound in which the metal ion is surrounded by more than one type of ligand is called the Heteroleptic complex. Various examples of heteroleptic compounds are,
These types of compounds have more than one type of donor atom. Read More, FAQs on Werner’s TheoryQ1: What is Werner’s Theory?Answer:
Q2: What is the Primary Valency according to Werner’s?Answer:
Q3: What is Secondary Valency?Answer:
Q4: What is a Double Salt?Answer:
Q5: What is a Coordination Complex?Answer:
Q6: Why do Coordination Compounds have color?Answer:
|
Reffered: https://www.geeksforgeeks.org
| Class 12 |
Type: | Geek |
Category: | Coding |
Sub Category: | Tutorial |
Uploaded by: | Admin |
Views: | 10 |