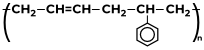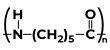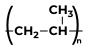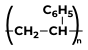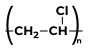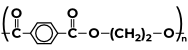
|
|
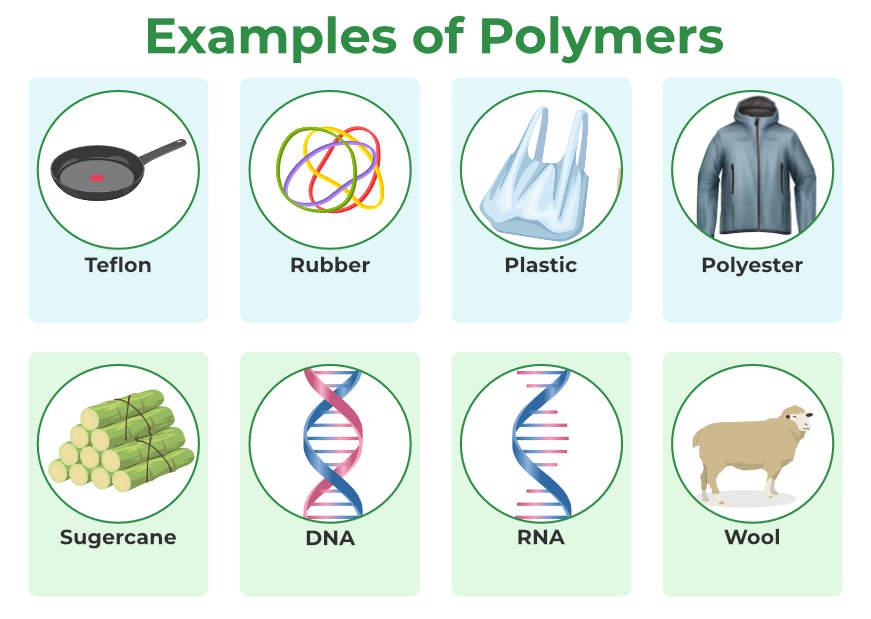
A Polymer is a very big molecule or complexly structured material made up of macromolecules, which are small, simple compounds that repeat themselves to make polymers. Both Synthetic Polymers as well as Natural Polymers play significant and relevant roles in daily life. As natural polymers, i.e., DNA and RNA, is the reason for life on earth, without DNA we can’t imagine life on earth. From well-known synthetic plastics like polystyrene to natural biopolymers like DNA and proteins that are essential to biological structure and function, polymers come in many shapes and sizes. Here are some examples of polymers in the figure given below. 
Table of Contents What are Polymers?The term “polymer” refers to an unknown amount of monomer units. When there are a lot of monomers, the mixture is sometimes referred to as a high polymer. Monomers of the same molecular weight, shape or chemical makeup are not required for polymers to form. One type of monomer makes up some natural polymers. However, the majority of polymers, both natural and manmade, are composed of two or more different kinds of monomers; these polymers are referred to as copolymers. Polymer Definition:
Classification of PolymersThe polymers are classified into various types based on different categories that are as follows: Classification of Polymers Based on the Source of AvailabilityOn the basis of the structure, polymers can be classified into three categories, which are as follows: Classification of Polymers Based on StructureOn the basis of the Source of occurrence, polymers can be classified into three categories, which are as follows: Classification of Polymers Based on PolymerizationOn the basis of the polymerization reaction used to form polymers, polymers can be classified into two categories, which are as follow: Classification of Polymers Based on MonomersOn the basis of monomers used to create polymers, polymers can be classified into two categories, which are as follows: Classification based on Molecular ForcesBased on the molecular forces between the constituent atom of polymer, polymers can be classified into two categories, which are as follows:
The Classification of Polymers is illustrated in the chart below: 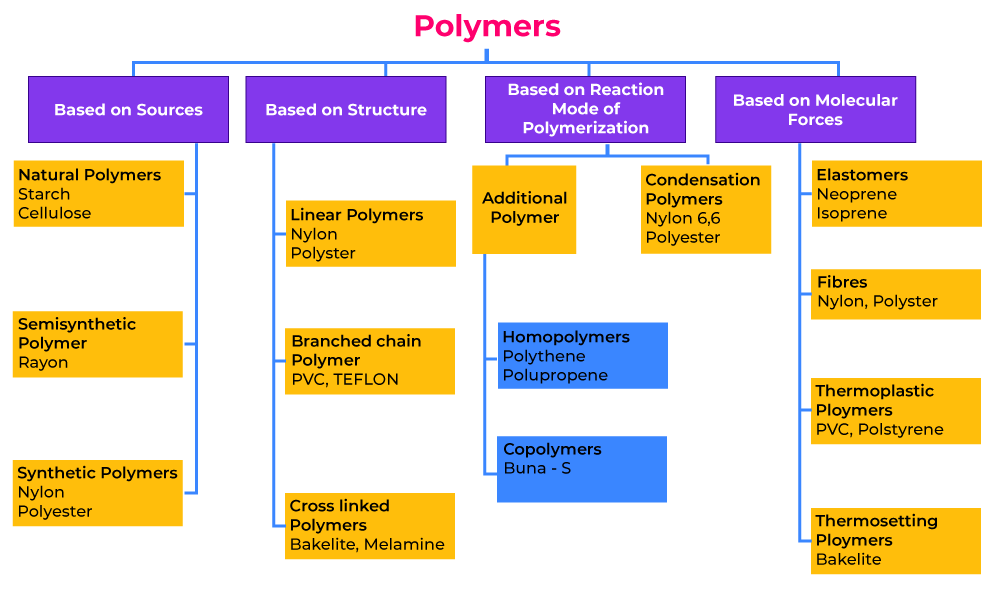
Structure of PolymersA hydrocarbon backbone makes up the majority of the polymers in our environment. Due to the tetravalent nature of carbon, a hydrocarbon backbone is a lengthy chain of linked carbon and hydrogen atoms. The hydrocarbon backbone polymers polypropylene, polybutylene, and polystyrene are a few examples. There are also polymers whose backbones contain other elements in place of carbon. For instance, the backbone of the repeating unit in nylon comprises nitrogen atoms. Characteristics of PolymersDepending on the use polymers can be made according to the desired properties and characteristics. Such as:
Some Polymers and Their MonomersA monomer is a kind of molecule with the ability to form long chains of chemical bonds with other molecules; a polymer is a chain made up of any number of monomers. Here is the list of Polymers with their monomers.
How are Polymers Made? – Polymerization ReactionsThe chemical reaction involved to produce a Polymer is called Polymerization Reaction. 
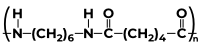
The method of producing polymers is called polymerization. Following processing, these polymers are used to create a variety of plastic items. Smaller molecules, or monomers, are joined chemically to form larger molecules, or macromolecules, during polymerization. A polymer is created by hundreds of these macromolecules working together. The molecules that make up the monomer could all be the same or could stand in for two, three, or more different substances. In order to create a product with specific physical characteristics that set polymers apart from substances made of smaller and simpler molecules, such as elasticity, high tensile strength, or the capacity to form fibers, typically at least 100 monomer molecules must be combined; frequently, thousands of monomer units are incorporated into a single polymer molecule. Types of Polymerization ReactionsThere are two types of polymerization reactions, which are as follows: Addition PolymerizationIn Addition Polymerization, small monomer units join together to form a giant polymer molecule. In each step of polymerization of this kind, the length of the polymer chain increases, that’s why it is also called Chain-Reaction or Chain-Growth Polymerization. For example, the formation of polyethylene from ethylene, the formation of PVC, and the formation of Teflon are some examples of the addition polymerizations. Condensation PolymerizationIn Condensation Polymerization, monomers including bifunctional groups such as diamines, -idols, dials, dicarboxylic acid, etc. undergo polymerization, and water, CO, and ammonia small molecules got eliminated to form polymers. The formation of nylon -6,6 is an example of Condensation Polymerization. Also Check: Step Growth or Condensation Polymerization and Types of Polymerization How to Calculate the Molecular Mass of Polymers?
A polymer is made up of repeating units. The monomer molecules were originally these repeating units. Polymer chains have different weights and lengths when they first form. The ability to describe the polymer structure is important. Any characterization of a polymer essential is either the weight-average or number-average molecular weight. Number Average Molecular Mass (Mn)Number mean or simple arithmetic mean, represents the weight of all molecules divided by the number of molecules.
Weight Average Molecular Mass (Mw)Weight average or simple arithmetic mean, represents the mass and molecular masses of all molecules divided by mass.
Degree of PolymerizationThe degree of Polymerization represents the number of monomeric units in a Polymer. It is defined as the ratio of the molar mass of polymer to the molar mass of the monomeric unit and is given as,
Uses of PolymersPolymers have a wide range of applications, such as:
Also Check: Polymers of Commercial Importance FAQs on PolymersQ1: Define vulcanization of rubber.Answer:
Q2: What are examples of biodegradable polymers?Answer:
Q3: What is Engineering Plastics?Answer:
Q4: What are Polymers used for?Answer:
Q5: Write some physical Properties of Polymers.Answer:
Q6: What are Biodegradable Polymers?Answer: These polymers break down after their purpose by bacterial decomposition process to result in natural byproducts. For example: Poly β-hydroxybutyrate -co-β-hydroxy valerate (PHBV). |
Reffered: https://www.geeksforgeeks.org
| Class 12 |
Type: | Geek |
Category: | Coding |
Sub Category: | Tutorial |
Uploaded by: | Admin |
Views: | 8 |