
|
|
A covalent bond is formed when two atoms exchange one or more pairs of electrons. Both the atomic nuclei of the shared atom together attract these electrons. When the difference between the electronegativities of two atoms is too small for an electron transfer to take place to create ions, a covalent bond is formed. Bonding electrons are collectively referred to as the electrons that are present between the two nuclei. The “bond” that holds the atoms in molecular units together is the bound pair. What is Covalent Bond?
Molecular bonds are another name for covalent bonds. The sharing of bonding pairs will ensure that the atoms achieve stability in their outer shell, similar to noble gas atoms. 
Elements with extremely high ionization energies are incapable of transferring electrons, and elements with extremely low electron affinity are incapable of absorbing electrons. The atoms of such elements tend to share electrons with atoms of other elements or atoms of the same element in such a way that both atoms achieve octet configuration in their respective valence shells and thus achieve stability. A covalent Bond refers to such an association formed by the sharing of electron pairs among different or similar kinds. Covalent Bonding in Carbon AtomA carbon atom has 4 electrons in its outermost orbit and it can logically have 3 possible ways of sharing its electrons. A carbon atom can gain 4 electrons to become C4- but it is not possible because it will be tough for 6 protons to hold 10 electrons and so the atom will become unstable. A carbon can lose 4 electrons to become C4+ but it would require a large amount of energy to remove out 4 electrons which again is not possible and the C4+ would have only 2 electrons held by the proton, which again is unstable. Thus carbon can not gain or donate electrons, so to complete its octet carbon atom can share all four electrons and form four covalent bonds. The image given below shows the Bonding in Carbon atoms. 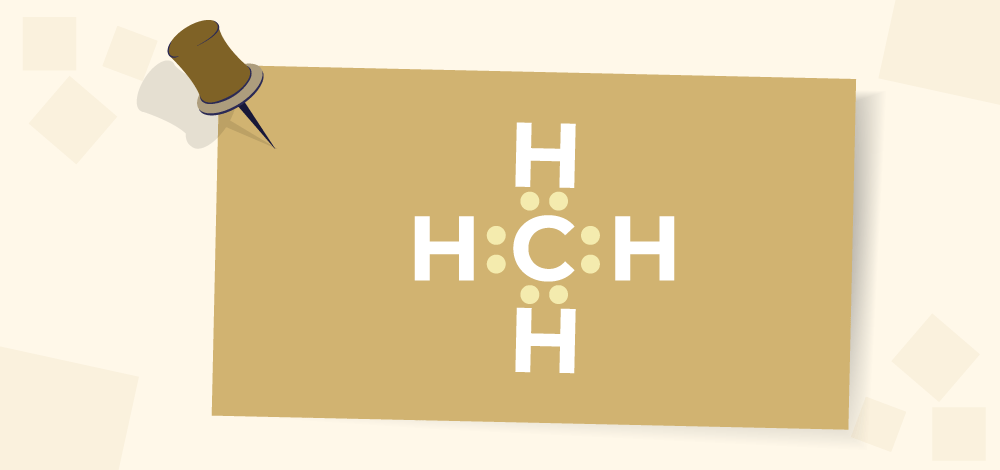
Covalent Bond ExamplesHere some important examples of covalent bonds are discussed in brief: 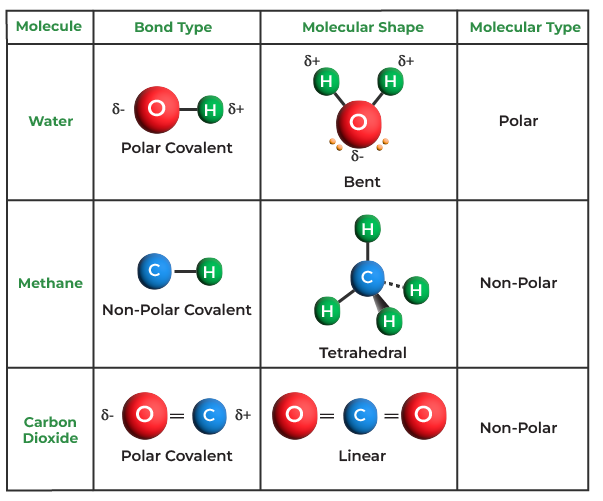
Covalent Bond in WaterCovalent bonds hold the hydrogen and oxygen atoms that makeup water molecules together. The incomplete outer shells of the hydrogen and oxygen atoms are where the hydrogen electron from the hydrogen spends the majority of its time. Two electrons (one from each hydrogen atom) are required to completely occupy the outer shell of oxygen, which contains six electrons but would be more stable with eight. This results in the well-known formula H2O. Both elements become more stable as a result of the sharing of electrons, which fill their respective outer shells. The image given below shows Covalent Bond in Water. 
Some Important Carbon Compounds with Covalent BondsMethaneThe outermost shell of carbon contains four electrons, and it requires four more to fill it. Each hydrogen atom contributes one toward these four, producing a stable outer shell of eight electrons. Despite not having the same electronegativity, hydrogen, and carbon are similar, and nonpolar bonds result. The outermost shell of the hydrogen atoms, which is full when it has two electrons, requires one electron for each atom. These substances combine to form a nonpolar covalent molecule by evenly distributing their electrons across the hydrogen and carbon atoms. Carbon DioxideCarbon Dioxide has two covalent bonds between carbon and oxygen atoms and its structure can easily be made using Lewis Dot methods. Carbon has four electrons in its outermost shell whereas oxygen has six electrons in its outermost shell, now carbon needs four electrons and oxygen needs two electrons to complete their octet. So carbon shares two electrons with both the oxygen atoms and hence all three atoms (One carbon atom and two oxygen atoms) achieve their stable configuration and thus, CO2 is formed. Properties of Covalent BondIf sharing a single electron pair between atoms does not satisfy an atom’s normal valence, the atoms may share more than one electron pair between them. Covalent bonds have the following properties:
What is the Octet Rule?Except for noble gases, all atoms have fewer than eight electrons in their valence shell which are called valence electrons. In other words, these atoms’ valence shells do not have stable configurations. As a result, they combine with one another or with other atoms to form stable electronic configurations. The tendency of atoms of different elements to achieve a stable configuration of eight electrons in their valence shells is the cause of chemical combination, and the principle of achieving a maximum of eight electrons in an atom’s valence shell is known as the octet rule. Conditions for Writing the Lewis Dot StructuresLewis Dot structure can easily explain the formation of the covalent bonds between two molecules. Some points we must remember for drawing Lewis Dot Structures are,
For Example, In the formation of an H2O molecule Hydrogen atom has one electron in its outermost shell and oxygen has six electrons in its outermost shell when two electrons of oxygen form a bond with two hydrogen atoms its octet is completed. Similarly, the stability of Hydrogen atoms is also achieved. Types of Covalent BondsDepending upon the number of shared electron pairs, the covalent bond can be classified into
Single BondsWhen only one pair of electrons is shared by the two participating atoms, a single bond is formed. It is denoted by a single dash (-). Despite having a lower density and being weaker than a double or triple bond, this type of covalent bond is the most stable. For example, an HCl molecule contains one Hydrogen atom with one valence electron and one Chlorine atom with seven valence electrons. In this case, hydrogen and chlorine form a single bond by sharing one electron. 
Double BondsWhen two pairs of electrons are shared by two participating atoms, a double bond is formed. It’s denoted by two dashes (=). Although double covalent bonds are much stronger than single bonds, they are also less stable. One carbon atom has six valence electrons and two oxygen atoms have four valence electrons in a carbon dioxide molecule, for example, Carbon shares two of its valence electrons with one oxygen atom and two with another oxygen atom to complete its octet. Because each oxygen atom shares two electrons with carbon, CO2 contains two double bonds.; 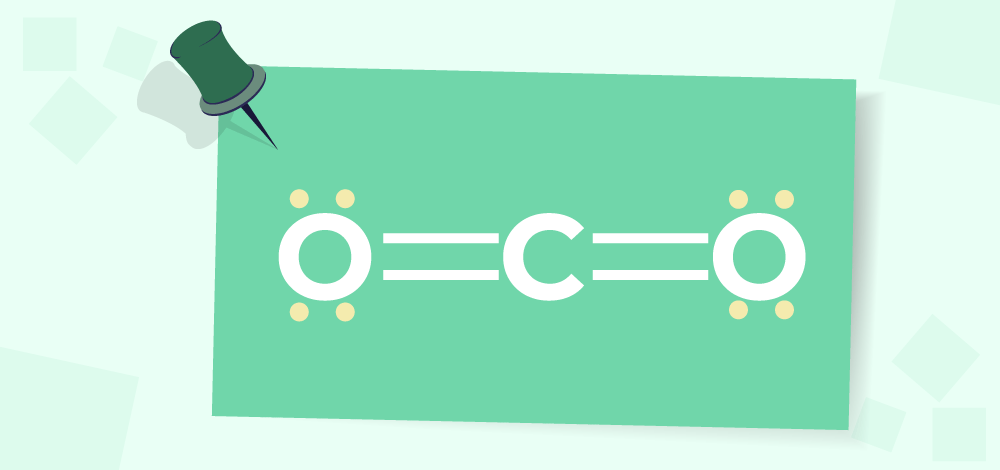
Oxygen-Molecule: Each oxygen atom in the formation of the oxygen molecule has six electrons in its valence shell. To complete their octet, each atom requires two more electrons. As a result, the atoms share two electrons to form the oxygen molecule. Because two electron pairs are shared, the two oxygen atoms form a double bond. Triple BondWhen three pairs of electrons are shared by two participating atoms, a triple bond is formed. Triple covalent bonds are the least stable type of covalent bond and are represented by three dashes. In the formation of a nitrogen molecule, for example, each nitrogen atom with five valence electrons contributes three electrons to form three electron pairs for sharing. As a result, a triple bond forms between the two nitrogen atoms. 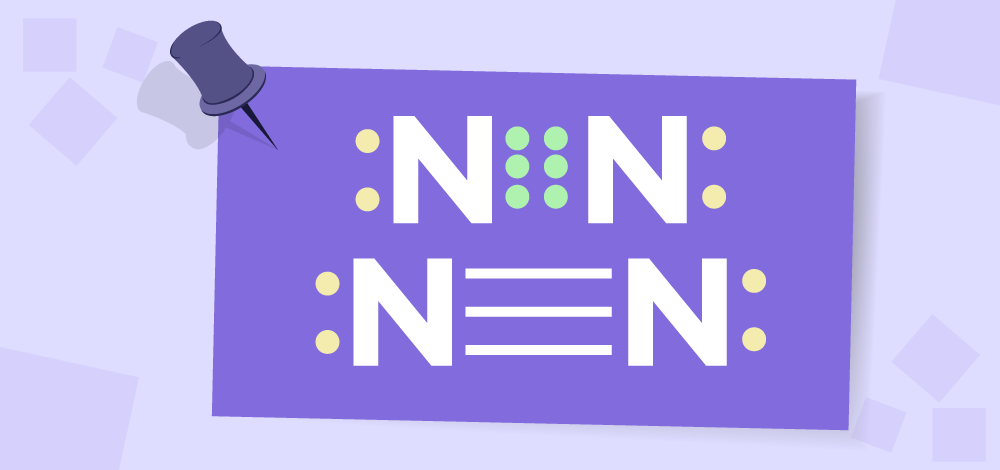
Polarization of Covalent BondsCovalent (sigma) Bonds also show some properties of electrovalent bonds this is achieved as the electron cloud between the two atoms in the sigma bond shifts towards one atom due to the difference in their electronegativity and the atom which attracts the electron cloud attains a partial negative charge and the other atom achieved a partial negative charge. Thus, due to these charges polarization happens and this results in the Polarization of Covalent Bonds For example, the sigma (covalent bond) in HCl is a polar covalent bond as Chlorine(Cl) has a higher electronegativity than Hydrogen(H). So the electron charge shifts towards Chlorine and it attains a partial negative charge. Similarly, Hydrogen attains a partial positive charge. Polar Covalent BondThis type of covalent bond exists when the electronegativity of combining atoms differs, resulting in unequal electron sharing. Electrons will be drawn to more electronegative atoms. The atoms’ electronegative difference is greater than zero but less than 2. As a result, the shared electron pair will be closer to that atom. For example, consider molecules that form hydrogen bonds due to an unbalanced electrostatic potential. The hydrogen atom in this case interacts with electronegative fluorine, hydrogen, or oxygen. 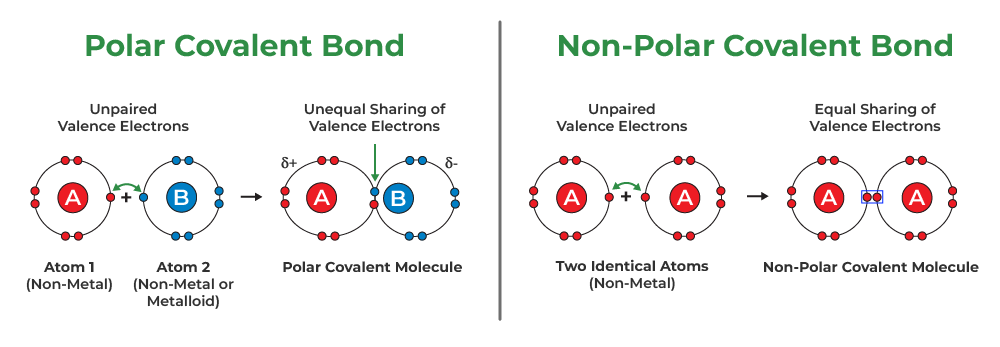
Nonpolar Covalent BondThis type of covalent bond is formed when two atoms share an equal number of electrons. The difference in electronegativity between two atoms is zero. It occurs whenever the atoms combined have a similar electron affinity. Nonpolar Covalent Bonds, for example, can be found in gas molecules such as hydrogen gas, nitrogen gas, and so on. Coordinate Covalent BondCoordinated or Dative Covalent Bond, this type of bond occurs when one of the atoms in the bond provides electrons for sharing. This is accomplished through the reaction of ammonia and boron trifluoride. Nitrogen has two free electrons, whereas boron lacks electrons. They complete their last shell with eight electrons by combining nitrogen and boron. 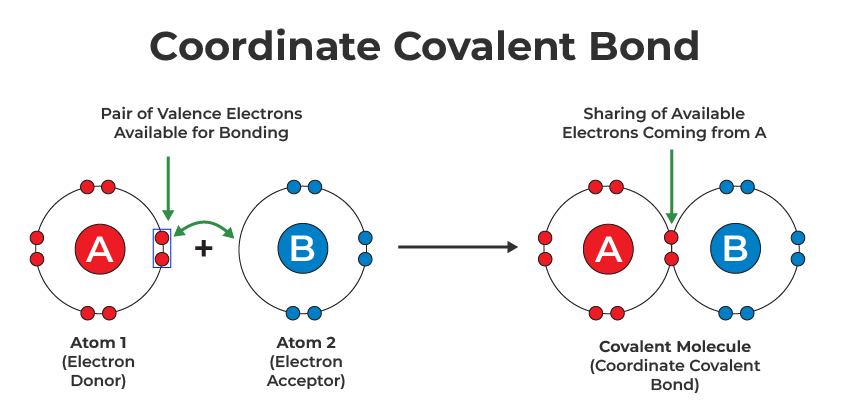
Difference between Ionic and Covalent BondThe major differences between Ionic Bonds and Covalent Bonds are discussed in the table given below,
Related Articles FAQs on Covalent BondsQ1: What are covalent bonds?Answer:
Q2: How are covalent bonds formed?Answer:
Q3: What are polar and nonpolar covalent bonds?Answer:
Q4: How many covalent bonds are in Cyclohexane?Answer:
Q5: How many covalent bonds are present in Ethane?Answer:
Q6: What are the total number of covalent bonds in CH4 (methane)?Answer:
Q7: Which molecule contains both polar and nonpolar covalent bonds?Answer:
|
Reffered: https://www.geeksforgeeks.org
| Class 11 |
Type: | Geek |
Category: | Coding |
Sub Category: | Tutorial |
Uploaded by: | Admin |
Views: | 10 |