
|
|
Bleaching Powder, also called Calcium Hypochlorite or Calcium Oxychloride, is a chemical compound which is used for various purposes. It has various uses but it is majorly used for its bleaching action, the bleaching action of Bleaching Powder is used to bleach clothes in laundries, and others. Bleaching powder is also used for various aspects such as disinfectants, cleaning potable water and others. In this article, we will read about Bleaching Powder, its properties, and others in detail. Bleaching PowderBleaching Powder, also known as chloride of lime, is solid in texture and yellowish-white in colour. Bleaching powder can be recognised by the smell of chlorine. Bleaching powder is generally used for the removal of colour, as indicated by its name. It is also known as a bleaching agent. The image given below shows the bleaching powder. 
Bleaching powder, when dissolved in water, forms an aqueous solution, which is basic in nature. Bleaching powder works on the principle of oxidation. The bleaching action of the bleaching powder is caused due to the presence of chlorine in it. A compound of calcium hydroxide exists, which is known as slaked lime reacts to form bleaching powder. Slaked Lime reacts with the chemical compound chlorine, it gives a compound, bleaching powder (calcium oxychloride) and gives water as a by-product. Bleaching powder is an inorganic compound with the formula Ca(OCl)2. The compound is soluble in water but due to the presence of impurities, the solution obtained by mixing this compound in water is not a clear solution.
Structure of Bleaching PowderThe structural formula of bleaching powder contains two atoms of chlorine. Among these two atoms, one of them is properly bonded to the atoms of the chemical compound Calcium. The other atom of Chlorine results in the formation of a bond with the atom of Oxygen. The image given below shows the structure of Bleaching Powder. 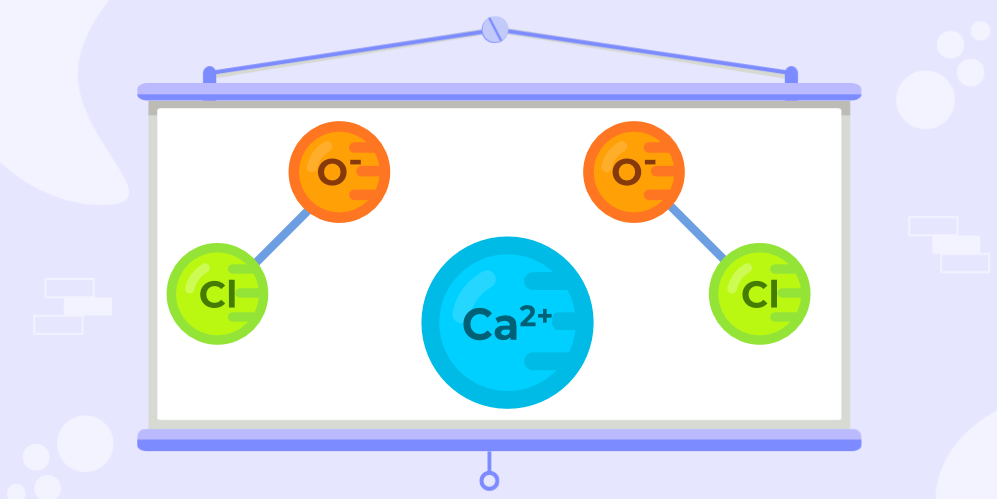
Preparation of Bleaching PowderThe manufacture of bleaching powder is carried out in Bachmann’s plant as follows, The entire system is prepared for the manufacturing of bleaching powder, containing a vertical cast-iron tower packed with a hopper at the top, two inlets near the base (one for chlorine and the other for hot air) and an exit for waste gases near the top. This tower is then fitted with shelves that are placed at varying heights with rotating rakes. The shelves are a total of eight in number. Slaked lime is introduced into the hopper. It eventually comes in contact with chlorine moving slowly in the upward direction. The bleaching powder is then collected at the base in the barrel area of the tower. The reaction used in the above reaction is, Slaked lime + Chlorine ⇢ Bleaching powder + Water
Important conditions to be maintained for preparation of the Bleaching Order
Properties of Bleaching PowderProperties of Bleaching Powder can be studied under two heading which includes,
Physical Properties of Bleaching PowderBleaching Powder is a pale yellowish powder that possesses a strong smell of chlorine. Its physical properties include,
Thereby, the chlorine that is produced as a result of the action of dilute acid on bleaching powder acts as a bleaching agent. Therefore, chlorine is the bleaching agent in bleaching powder. Chemical Properties of Bleaching PowderVarious Chemical Properties of Bleaching Powder include,
The chlorine released in various reactions of bleaching power releases chlorine gas which is used as a bleaching reagent. pH of Bleaching PowderBleaching Powder is basic in nature and the bleaching powder which is generally used for domestic purposes has a pH value of 11. Thus, bleaching powder has an irritating nature when coming in contact with human skin. Concentrated Bleaching Powder, which has a high concentration (10-15% ) of sodium hypochlorite, has a pH value of 13. Thus, it is highly alkaline in nature. Uses of Bleaching PowderBleaching Powder has the following uses:
Read More FAQs on Bleaching PowderQuestion 1: What is Bleaching Powder?Answer:
Question 2: What are the uses of Bleaching Powder?Answer:
Question 3: What is the chemical name for Bleaching Powder?Answer:
Question 4: What is the formula for bleaching powder?Answer:
Question 5: Is Bleaching Powder Acidic or Basic?Answer:
Question 4: What is the pH value of bleaching powder?Answer:
Question 5: Why does bleaching powder act as an oxidising agent?Answer:
Question 6: What are some harmful effects of bleaching powder?Answer:
|
Reffered: https://www.geeksforgeeks.org
| Class 10 |
Type: | Geek |
Category: | Coding |
Sub Category: | Tutorial |
Uploaded by: | Admin |
Views: | 9 |