|
|
Isotopes are those atoms that are having the same atomic number or the same position in the modern periodic table but with different atomic masses. This difference in atomic mass arises from the different numbers of neutrons present in the nucleus of the atom. As they lie in the same position on the periodic table they almost have similar chemical properties but different physical properties due to the difference in atomic mass. Other than Isotopes, there are isobars, and isotones are also defined based on the number of subatomic particles in the atom. Table of Content Isotopes Definition
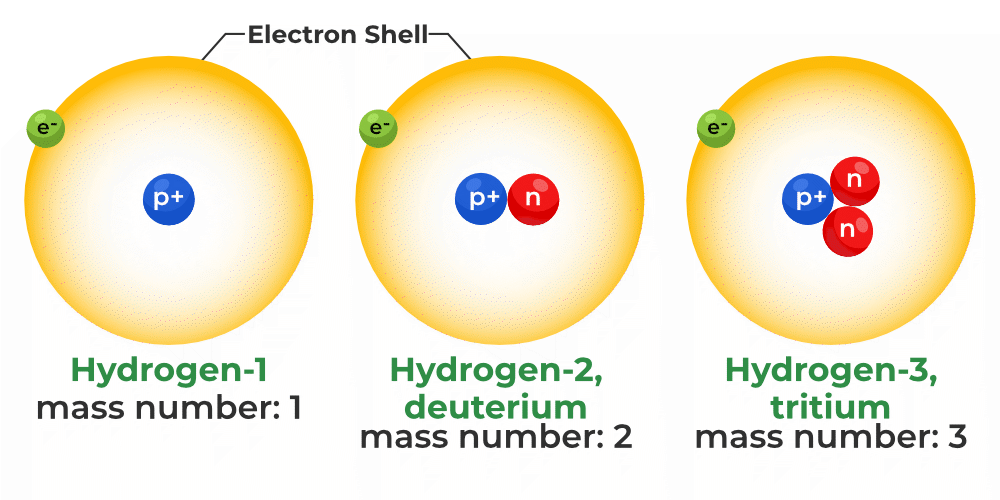
There are several isotopes found in nature in the stable form but some are found in the unstable form(radioactive). The most stable and common isotopes of an element are often one or two of them. Due to their similar proton and electron numbers, different isotopes of an element usually share the same physical and chemical characteristics. Examples of IsotopesThere are a lot of examples of the nature of Isotopes, some of which are as follows: Isotopes of HydrogenHydrogen has three naturally occurring Isotopes. Isotopes of Hydrogen are 1H1 Protium, 1H2 Deuterium, and 1H3 Tritium, but in a lab environment, we can create more isotopes with different numbers of neutrons in the nuclei of it. These synthetic isotopes of Hydrogens are all radioactive and have a half-life of the order of yotta seconds. 
Isotopes of CarbonCarbon-13 and Carbon-14 are both isotopes of carbon, one with 7 neutrons and one with 8 neutrons (but both with 6 protons). Carbon-12 is a stable isotope, while carbon-14 is a radioactive isotope (radioisotope). Other than this, many synthetic carbon isotopes are synthesized in a lab environment and can only sustain for a very brief period of time(order of 200 ms). 
Isotopes of OxygenThere are only three stable isotopes of oxygen which are 16O, 17O, and 18O. Other than this oxygen nuclei with a different number of neutrons such as 3, 4, 5, 6, 7, etc. can be synthesized in labs but they are not stable in nature and only can exist in the lab environment.
Learn More,
Types of IsotopesThere are two types of Isotopes based on their stability, stable and unstable (radioactive) isotopes, which are explained as follows:
Now let’s learn about them in detail. Stable IsotopesStable isotopes are isotopes with extraordinarily long half-lives (half-life is defined as the time it takes for a substance to decompose and reach a concentration or weight that is half, or 50%, of its initial concentration or weight). Examples of Stable Isotopes are Carbon (Carbon-12, Carbon-13, and Oxygen (Oxygen-16, Oxygen-17, and Oxygen-18). Radioactive IsotopesSome isotopes have extremely short half-lives and degrade quickly, emitting radioactive waves as they do so. These are known as radioactive isotopes. Isotopes that are radioactive include Tritium, Carbon-14, etc. Primordial IsotopesThe nuclides that have been around since the creation of our solar system are known as primordial nuclides. On Earth, there are 339 naturally occurring isotopes in total, 286 of which are Primordial Isotopes. What are Radioactive Isotopes?Radioactive Isotopes aka Radioisotopes, are unstable isotopes of an element. These isotopes emit radiation in the form of radioactive decay. Most radioisotopes are created through nuclear reactions, such as those that occur in nuclear reactors or particle accelerators. But some radioisotopes occur in nature such as carbon-14 and uranium-238. These isotopes go through the process of radioactive decay to form more stable nuclei and emit alpha, beta and gamma radiation during the decay. These emissions of radiation took place over a very short period of time or over millions of years, depending on the half-life of the isotope. Isotopes of UraniumUranium is a naturally occurring radioactive element found in nature that has many isotopes but no isotope is stable in nature. But some of these radioactive isotopes have a significant half-life (in order of million years). Also, it has two primordial isotopes i.e., U-235 and U-238, out of which U-238 is the most abundant isotope on earth and has the most half-life as well. Therefore it is used in research and nuclear applications. Application of Radioactive IsotopesThe following are the important uses of Radioactive Isotopes:
Uses of IsotopesSome of the important uses or applications of Isotopes are:
Differences between Isotopes and IsobarsThere are several differences between Isotopes and Isobars, which are as follows:
Read More, Sample Question on IsotopesQuestion 1: What are the Applications of Isotopes? Answer:
Question 2: Isotopes have the same chemical properties while isobars do not have the same. Why? Answer:
FAQs on IsotopesWhat are Isotopes?
What are Isotopes of Hydrogen?
Which Isotope of Hydrogen is Radioactive?
Who is Credited for the Discovery of Isotopes?
Which Radioactive Isotope is used in Treatment of Cancer?
|
Reffered: https://www.geeksforgeeks.org
| Class 9 |
Type: | Geek |
Category: | Coding |
Sub Category: | Tutorial |
Uploaded by: | Admin |
Views: | 8 |
