|
|
Benzene is aromatic compound which act as precursor to derive other compounds. Reactions of benzene involve the substitution of a proton by other groups. Electrophilic aromatic substitution is a method of derivatizing benzene. The most common example of this reaction is the ethylation of benzene. Different important reactions of benzene include sulfonation, chlorination, nitration, and hydrogenation. The activating or deactivating effect of substituents on the benzene ring determines the reaction’s direction and the ring’s reactivity. In this article, we will learn about the different reactions of benzene, along with basic introduction of benzene and its structure. Table of Content What is Benzene?Benzene is a colourless or light yellow, highly flammable liquid with a sweet odor. It consists of six carbon atoms arranged in a planar hexagonal structure, forming a ring with one hydrogen atom attached to each carbon. Benzene is an aromatic hydrocarbon. It possesses unique chemical stability due to the delocalization of its electrons across the ring. This stability makes benzene a versatile building block for various industrial applications, including manufacturing plastics, resins, synthetic fibers, lubricants, dyes, detergents, drugs, and pesticides. Structure of BenzeneBenzene is a planar molecule of six carbon atoms arranged in a hexagonal ring, with one hydrogen atom attached to each carbon atom. Each carbon-carbon bond in benzene exhibits equal bond lengths, approximately 1.39 angstroms (Å), which is longer than a standard single covalent bond (approximately 1.47 Å) but shorter than a double bond (approximately 1.34 Å). This unusual bond length arises from the delocalization of electrons over the entire ring, creating a system of alternating single and double bonds, referred to as a resonance hybrid or a delocalized π-electron cloud. Reactivity of BenzeneThe nature of its substituents influences the reactivity of benzene in substitution reactions. Substituents can activate or deactivate the benzene ring, affecting its reactivity in electrophilic aromatic substitution (EAS) reactions. Electron-donating groups, such as -OH and -CH3, activate the benzene ring, increasing its reactivity towards EAS. In contrast, electron-withdrawing groups, such as -NO2 and -COOH, deactivate the benzene ring, decreasing its reactivity in EAS. The substituents’ nature also influences the substitution position in the benzene ring. Activating groups generally direct substitutions to the ortho and para positions, while deactivating groups direct substitutions to the meta positions. Reactions of BenzeneBenzene undergoes various reactions, particularly electrophilic aromatic substitution (EAS) reactions. Notably, some of the significant reactions include: Electrophilic Aromatic Substitution (EAS)
Electrophilic Addition Reactions
Benzene Reduction
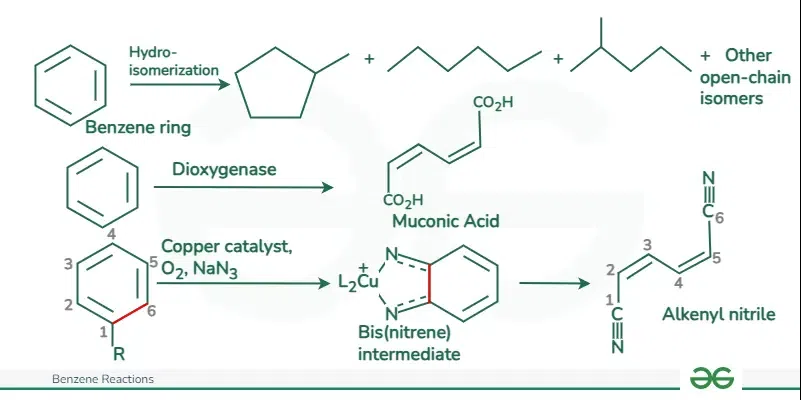
Benzene Ring Opening Reaction
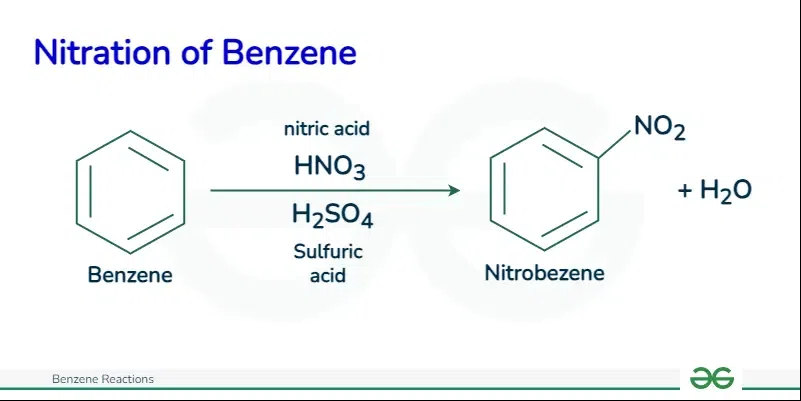
Electrophilic Substitution ReactionBenzene undergoes Electrophilic Susbtitution Reaction. In these reactions, an electron deficit species that need an electron also called electrophile replaces an atom or group of atoms in benzene to form another compound. Electrophilic Substitution Reaction includes the following reactions let’s discuss them one by one. Nitration of BenzeneThe nitration of benzene is an electrophilic substitution reaction between benzene and a mixture of concentrated nitric acid and concentrated sulfuric acid.
The reaction of nitration of benzene is shown mentioned below:
The mechanism for the nitration of benzene can be summarized as follows:
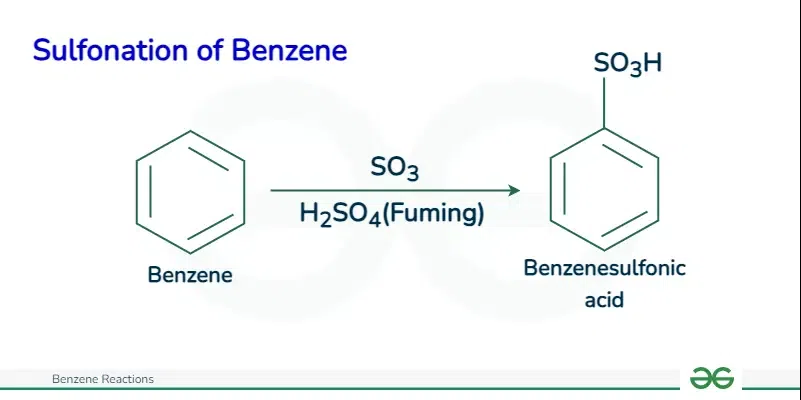
Sulfonation of BenzeneThe sulfonation of benzene proceeds via an electrophilic aromatic substitution mechanism, specifically utilizing sulfur trioxide (SO3) or fuming sulfuric acid (oleum, H2S2O7) containing SO3 as the electrophile.
Here is a step-by-step description of the mechanism:
Here is a simplified representation of these steps:
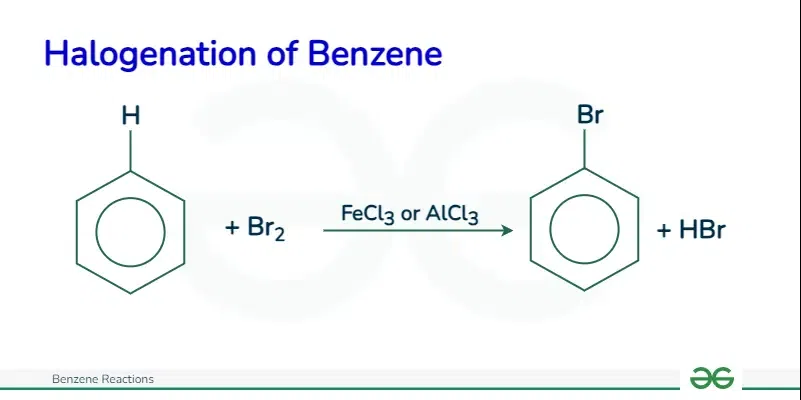
Halogenation of BenzeneThe halogenation of benzene involves the substitution of a hydrogen atom on the benzene ring with a chlorine or bromine atom. This reaction occurs in the presence of a catalyst such as aluminum chloride or iron. The catalyst helps activate the halogen to become a strong electrophile.
For example, chlorobenzene is formed when benzene reacts with chlorine in the presence of aluminum chloride. The specific reactions for chlorination and bromination of benzene are as follows:
The mechanism of electrophilic aromatic substitution between benzene and chlorine or bromine involves several steps:
Electrophilic Addition ReactionElectrophilic addition reactions involve the interaction between an electrophile and a substance containing a pi bond, forming new sigma bonds. They are crucial in transforming alkenes and alkynes into various functional groups. The process typically occurs in two main steps:
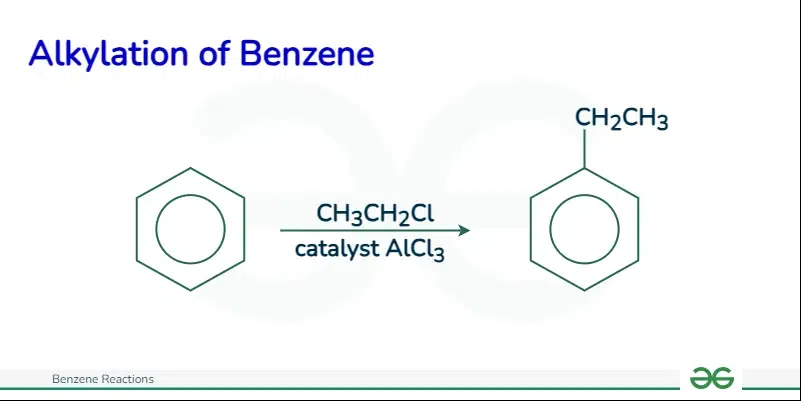
Friedel Craft AlkylationIn this reaction, an alkylating agent, like an alkyl halide, transfers an alkyl group to the aromatic ring in the presence of a strong Lewis acid catalyst, such as aluminum chloride.
The mechanism of Friedel-Crafts alkylation involves three main steps:
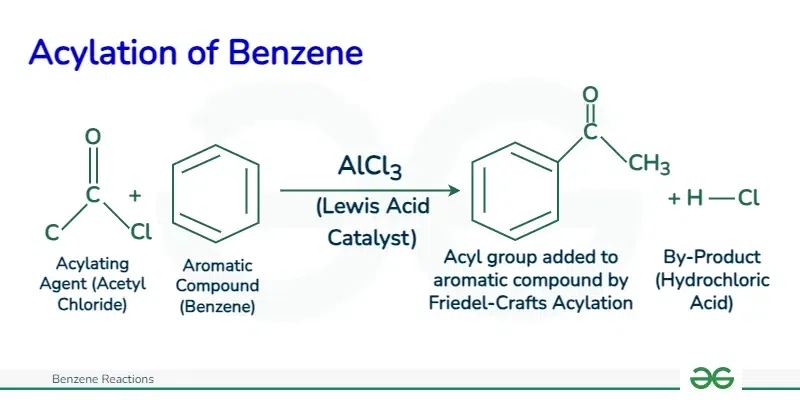
Friedel Craft AcylationFriedel-Crafts acylation is a reaction that involves the acylation of aromatic rings using acyl halides and Lewis acids, such as aluminum trichloride, as catalysts.
The mechanism of Friedel-Crafts acylation involves three main steps:
Benzene ReductionBenzene reduction can be achieved through various methods, including catalytic hydrogenation, Birch reduction, and Friedel-Crafts acylation followed by reduction. Catalytic hydrogenation of benzene requires forcing conditions, such as high heat and hydrogen pressure, to reduce the aromatic ring selectively without affecting other functional groups. Under milder conditions, the double bond of an alkene can be reduced without reducing the aromatic ring. Benzene HydrogenationBenzene hydrogenation is a chemical reaction in which benzene (C6H6) reacts with hydrogen (H2) in the presence of a suitable catalyst to form cyclohexane (C6H12). The general equation for benzene hydrogenation is:
The reaction proceeds under high pressure and elevated temperature to increase the rate of hydrogenation. This reaction requires a catalyst such as platinum (Pt), palladium (Pd), nickel (Ni), and ruthenium (Ru) to facilitate the reaction. Benzene Ring Opening ReactionThe ring-opening reaction of benzene is not common or typical, as benzene is known for its stability and resistance to ring-opening. The aromaticity of benzene, resulting from its delocalized pi electrons, makes it highly resistant to ring-opening reactions.
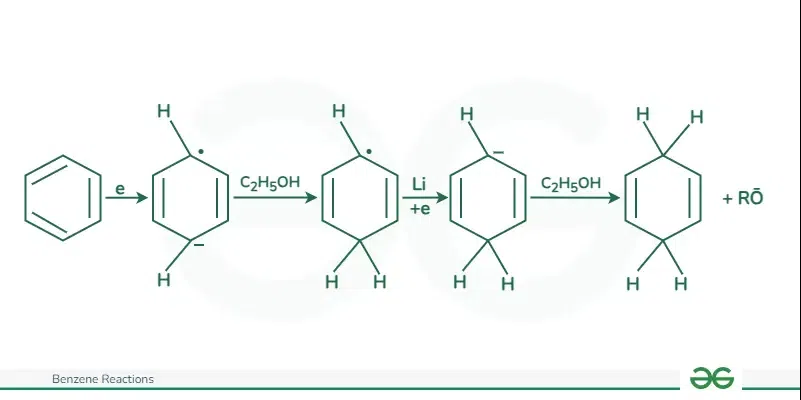
Birch ReductionThe reduction of benzene can be achieved through Birch reduction, which involves using an electron-rich solution of alkali metals, usually lithium or sodium, in liquid ammonia. The mechanism of the Birch reduction is given below:
Benzene OxidationThe oxidation of benzene is a complex process because of the stability and resistance of the aromatic ring to oxidation. However, benzene can be oxidized under extreme conditions or with specific catalysts to form various products. There are some examples of oxidation of benzene are
Electrophilic Aromatic SubstitutionElectrophilic aromatic substitution (EAS) occurs through a two-step process:
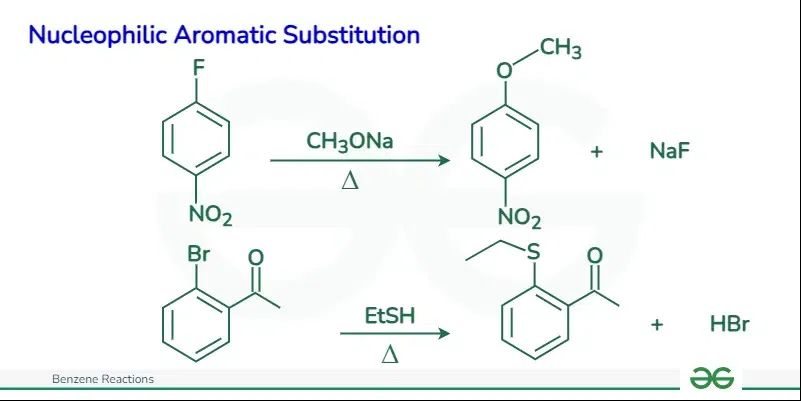
This mechanism contrasts with nucleophilic aromatic substitution (NAS), where the aromatic ring acts as an electrophile and forms a negatively charged intermediate before the departure of the leaving group. Nucleophilic Aromatic SubstitutionNucleophilic aromatic substitution (NAS) is a substitution reaction in organic chemistry in which a nucleophile replaces a leaving group on an aromatic ring. The reaction proceeds through a negatively charged intermediate, and the presence of electron-withdrawing groups on the aromatic ring accelerates the reaction. The reaction is different from a typical SN2 reaction because it occurs at a trigonal carbon atom (sp2 hybridization)
The mechanism steps for nucleophilic aromatic substitution (NAS) involving benzene can be summarized as follows:
More Benzene ReactionsApart from the above mentioned reactions, there are more some reactions which is essential in chemistry such as Gatterman Koch reaction, mercuration of benzene, ozonolysis of benzene etc. Gattermann-Koch synthesisThe Gattermann-Koch reaction is a method for producing benzaldehyde from benzene using carbon monoxide (CO) and hydrochloric acid (HCl) in the presence of anhydrous aluminum chloride (AlCl₃) Step 1: Formation of formyl chloride
Step 2: Formation of electrophile (H-C≡O)
Step 3: Formation of benzaldehyde
Gattermann Aldehyde ReactionGattermann aldehyde synthesis is an organic reaction used to synthesize aromatic aldehydes. The reaction involves the conversion of aromatic compounds into aldehydes using hydrogen cyanide, hydrogen chloride, and a Lewis acid such as aluminum chloride. Step 1. Formation of Formimino Chloride
Step 2. Formation of Electrophile
Step 3. Attack of Electrophile on Benzene Ring
Step 4. Hydrolysis of Benzylamine
Mercuration of BenzeneThe mercuration of benzene involves adding a mercury-containing group to the benzene ring. This reaction is typically carried out using mercuric acetate or mercuric chloride in the presence of a Lewis acid catalyst such as aluminum chloride. The general reaction for the mercuration of benzene :
Blanc ChloromethyationThe Blanc chloromethylation is also called the Blanc reaction. It is a chemical process that introduces a chloromethyl group on aromatic rings. This reaction involves treating aromatic compounds with formaldehyde in the presence of a Lewis acid catalyst such as zinc chloride. The general reaction for blanc-chloromethylation
Combustion Reaction of BenzeneThe combustion reaction of benzene involves the reaction of benzene (C₆H₆) with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O). The balanced chemical equation for the combustion of benzene is as follows:
This balanced equation indicates that one mole of benzene reacts with 15.5 moles of oxygen to produce 6 moles of carbon dioxide and 3 moles of water. Ozonolysis of BenzeneThe ozonolysis of benzene involves reacting with ozone under specific conditions to form organic compounds. The conditions for the ozonolysis of benzene are as follows:
Ozonolysis is a reaction where an alkene or alkyne reacts with ozone to form organic compounds by replacing the multiple carbon-carbon bonds with a double bond to oxygen. In the case of benzene, ozonolysis leads to the formation of glyoxal through the production of benzene triozonide as an intermediate. Benzene with Metal ComplexMetal complexes of benzene have been extensively studied in organometallic chemistry. These complexes are derivatives of benzene, where a transition metal atom replaces a CH center. The metal complexes of benzene can be classified into two categories:
Disubstituted Benzene ReactionsDisubstituted Reactions of benzene refer to reactions where two substituents are introduced onto the benzene ring. These reactions can occur through various mechanisms, including electrophilic aromatic substitution, nucleophilic aromatic substitution, or radical substitution. The general mechanism for a disubstituted reaction of benzene via electrophilic aromatic substitution involves two sequential electrophilic substitution reactions.
Benzylic Position and Impact on Benzene ReactivityThe benzylic position refers to the carbon adjacent to an aromatic ring, particularly in benzene. This position is characterized by increased reactivity due to the resonance stabilization of the benzylic carbocation, which results from the delocalization of the positive charge onto the aromatic ring. The benzylic position plays a significant role in many types of reactions such as:
These reactions are influenced by factors such as the electronic nature of the substituents attached to the aromatic ring, which can affect the overall reactivity of the benzylic position. Uses of Benzene ReactionsBenzene Reactions are important in chemistry for formation of numerous compounds. Here are few uses of benzene reactions:
Benzene Reactions FAQsWhat is the molecular formula of benzene?
What are the three types of substitution reactions of benzene?
What are the nucleophilic aromatic reactions?
Why is the reaction of benzene essential in organic chemistry?
What are the differences between electrophilic substitution and electrophilic addition reactions?
What are the differences between electrophilic substitution and nucleophilic substitution reactions?
What is birch reduction?
What are the applications of benzene reaction?
|
||||||||
Reffered: https://www.geeksforgeeks.org
| Class 12 |
Type: | Geek |
Category: | Coding |
Sub Category: | Tutorial |
Uploaded by: | Admin |
Views: | 16 |